Which of the Following Statements About Polar Molecules Are True
Have high melting point c. Which is true of a polar covalent bond.
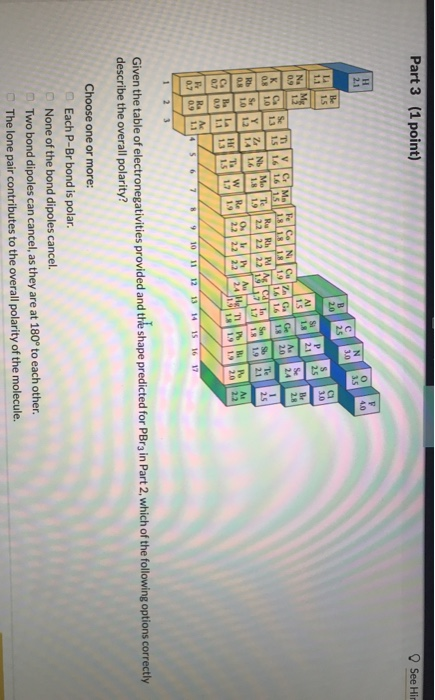
Solved 1 Point Part 1 Which Of The Following Statements Chegg Com
- Get Solutions for your doubts here at BYJUS.

. Previous The half-life of. A The centre of gravity of electrons and protons coincide. The dipoles are unequally.
A polar bond is a type of chemical bond. This eventually cancels out giving a zero net force and hence the molecule cannot be polar. Previous Devon a vice president.
I didnt take chemistry in high school but I did learn basic research in. And see if each comment is true or false. CCl4 is a polar molecule.
Not all molecules having polar bonds are polar. Which of the following statements could be true regarding polar molecules. Look up each type ie linear molecules lone etc.
A polar molecule may have one or more lone pairs. Have low surface tension d. Which of the following statements is not true for polar molecules.
A polar molecule may have one or more lone. Which of the following statements is. Which of the following statements is true about nonpolar molecules a.
Choose one or more. For a polar molecule which of the following statements is true. A water molecule is held together by two single polar covalent bonds.
CCl4 is a polar molecule. Statement 1 is false while statement 2 is true. Molecules consists of polar bonds can be nonpolar.
A polar molecule will not contain polar bonds. Choose one or more. They dissolve with non-polar compounds C.
Which of the following statements could be true regarding polar molecules. A polar molecule is attracted to other molecules because of electrostatic. - They are attracted to other polar molecules - They have the same number of protons and electrons.
All polar molecules have dipole moments. A polar molecule will not contain polar bonds. A polar molecule may have one or more lone pairs.
An example is linear carbon dioxide molecule CO2. Which of the following statements could be true regarding polar molecules. Statement 1 is true while.
1 point Part 1 Which of the following statements could be true regarding polar molecules. The given statement is False. Look up each type ie linear molecules lone etc.
Which of the following statements could be true regarding polar molecules. O A polar molecule will never contain nonpolar bonds. Which of the following statements could be true regarding polar molecules.
The statement there must be an odd number of polar bonds so that their polarities not cancel regarding polar molecules is FALSE. These are true and false questions and I want to make sure that I got them right. Which statement is true about Polar Molecules.
They dissolve with ionic compounds B. A molecule with a polar bond is always polar. Polar covalent bonding is a type of chemical bond where a pair of electrons is unequally shared between two atoms.
The bond dipoles in a polar. Choose one or more. They have similar charges on.
A polar molecule will not contain polar bonds. In order to have a polar molecule there must be unequal distribution of the negatively charged electrons in the orbitals of the molecule. Have high boiling point b.
A polar molecule has an uneven distribution of electron density. Molecules with identical bond angles and identical peripheral atoms are symmetrical. Which of the following statements about polar molecules are true.
Molecules with identical bond angle and identical peripheral atoms are asymmetrical. They are symmetrical A. All polar molecules have oppositely charged regions.
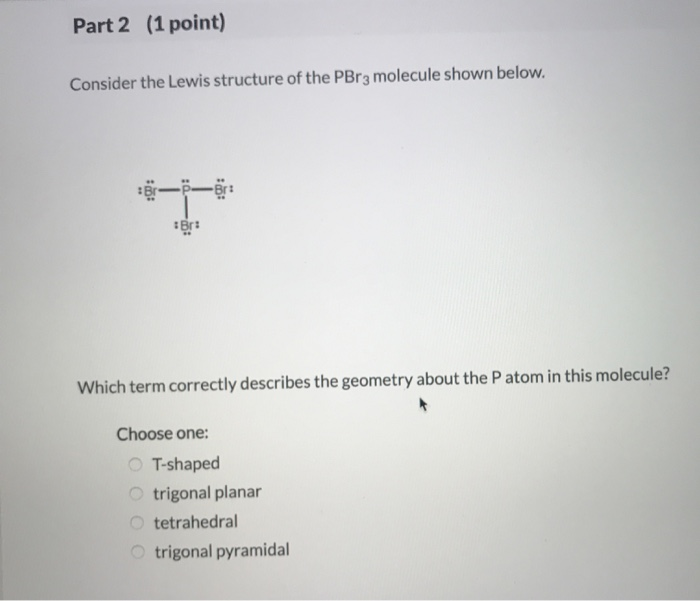
Solved 1 Point Part 1 Which Of The Following Statements Chegg Com

Polar And Non Polar Molecules Tmjh 8th Grade Science

Solved 1 Point Part 1 Which Of The Following Statements Chegg Com
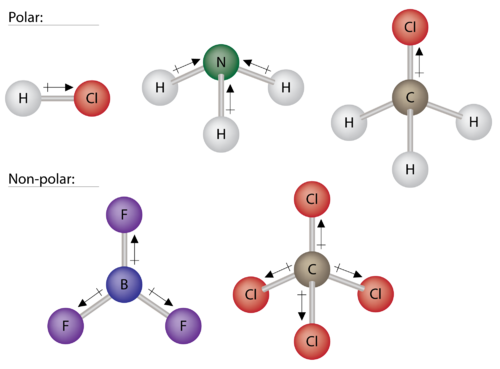
No comments for "Which of the Following Statements About Polar Molecules Are True"
Post a Comment